Criteria for the safety assessment of food cultures
Safety assessment and characterisation of food cultures
Scope and disclaimer: This document is meant as a set of recommended guidelines agreed upon by EFFCA members for the safety assessment and characterisation of food cultures (FC). However, a case-by-case assessment may remain relevant[1].
Definition: Food cultures (FC) are safe live bacteria, yeasts or filamentous fungi (moulds) used in food production which are in themselves a food ingredient. FC preparations are formulations, consisting of concentrates (> 10^8CFU/g or ml for bacteria and yeasts and > 10^7CFU/g for filamentous fungi) containing one or more live and active microbial strains of one or more microbial species, including unavoidable metabolites and media components carried over from the fermentation and components (e.g., carbohydrates, organic acids, minerals, vitamins) which are necessary for their survival, storage and to facilitate their application in the food.
FC includes, but is not limited to the terms: starter cultures, dairy starter, yoghurt starters, ripening cultures, meat cultures, sausage starter, wine cultures, plant-based starters, malolactic cultures, sourdough starter, probiotics, lactic acid bacteria, etc. For further explanations, see EFFCA paper on the definition of food cultures.[2]
Safety assessment - references:
The 2018 EFSA Guidance on the characterisation of microorganisms used as feed additives or as production organisms was considered as a basis to develop the criteria for the safety assessment and characterisation of FC (Fig. 1).
Criteria [3]:
- Species identification - is carried out by preferably using whole genome sequencing (WGS, e.g., according to EFSA 2021) and/or other up-to-date method.
- QPS qualification - for strains belonging to QPS species, qualifications mentioned in the QPS list (Appendix E) are fulfilled (EFSA BIOHAZ Panel, 2022) 2.
- Antimicrobial[4] susceptibility - performed according to the method described in EFSA 2018 and 2021 guidelines.
- Antimicrobial production - for strains not belonging to a QPS species except those already known not to produce relevant antimicrobials. Genomic screening for biosynthetic gene clusters involved in the production of antimicrobial substances by using up-to-date databases (see Annex I of this document). Annex I implies that only antibiotics (HIAs and CIAs) are in scope, which is being covered by the WGS analysis by searching for sequences of concern. In case of presence of these clusters, antimicrobial production should be evaluated by assessing of the inhibitory activity of culture supernatants against reference strains known to be susceptible to a range of antibiotics (e.g., Escherichia coli ATCC 25922, Pseudomonas aeruginosa ATCC 27853, Staphylococcus aureus ATCC 25923, Enterococcus faecalis ATCC 29212 and Bacillus subtilis ATCC 6633 or other reference strains, EUCAST, 2015; FAO, 2006).
- Toxigenicity and pathogenicity - for non-QPS species up to date literature searches are done to collect information relating to toxigenicity and virulence for humans, including history of use of the species, strain or any close relative. Targeted genomic blast searches are then performed for genes of concern identified in literature (EFSA 2018, EFSA 2021). If species are known to produce a toxic compound, analyses should be made to exclude their presence or demonstrate that their concentration in the specific application is of no concern[5].
- Absence of virulence factors – for prokaryotic microorganisms based on interrogation of WGS against relevant databases.
Figure 1. Decision tree on how to assess safety of strains used in food cultures
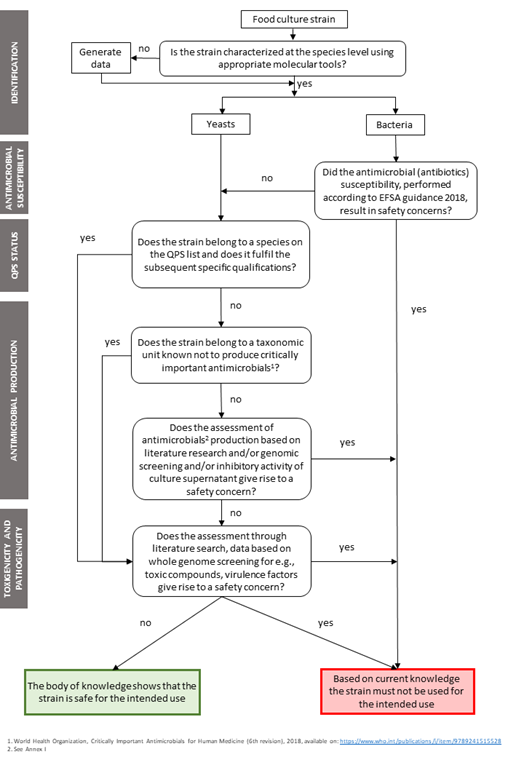
* List of QPS-recommended biological agents for safety risk assessments carried out by EFSA (EFSA, 2022)
** Guidance on the characterization of microorganisms used as feed additives or as production organisms (EFSA, 2018) and EFSA statement on the requirements for whole genome sequence analysis of microorganisms intentionally used in the food chain (EFSA, 2021) additionally for yeast strain, absence of resistance to antimycotics used for medical treatment of yeast infections in cases where viable cells are added to the food or feed chain
*** Inventory of microbial food cultures with safety demonstration in fermented food products (Bourdichon et al., 2022)
_______________________
References
EFSA, 2018. Guidance on the characterisation of microorganisms used as feed additives or as production organisms. EFSA Journal 2018;16(3):5206.
EFSA, 2021. EFSA statement on the requirements for whole genome sequence analysis of microorganisms intentionally used in the food chain. EFSA Journal 2021;19(7):6506.
EFSA BIOHAZ Panel, 2022. Updated list of QPS-recommended biological agents for safety risk assessments carried out by EFSA. Zenodo. doi: 10.5281/zenodo.6902983.
Bourdichon, F. et al., Bulletin of the IDF No. 514/2022, Inventory of microbial food cultures with safety demonstration in fermented food products, 1-175.
*****
Annex I – antimicrobial production – explanations
There is no definition from EFSA of what antimicrobials are, but EFSA is looking at the WHO definitions, as follows:
Antimicrobial: An agent or substance, derived from any source (microorganisms, plants, animals, synthetic or semisynthetic) that acts against any type of microorganism: bacteria (antibacterial), mycobacteria (antimycobacterial), fungi (antifungal), parasite (antiparasitic), and viruses (antiviral). All antibiotics are antimicrobials, but not all antimicrobials are antibiotics. The scope of this report is limited to the antibacterial antimicrobials.
Antibiotic: An agent or substance that is produced from microorganisms that can act against another living microorganism. Antimicrobial substances that are synthetic, semisynthetic, or those derived from plants or animals, are therefore, by strict definition, not considered antibiotics.
When considering “antimicrobial production”, EFSA intends those compounds that could be produced by microorganisms, and that are used for treating human/animals diseases/infections. Below a document from WHO, where one can find tables describing which are those compounds that would fall into this category. Therefore, when doing a safety evaluation of FC/bacteria, the idea would be to make sure that the strain is not able to produce any of the listed compounds. Only then would we be able to claim that the strain “does not produce antimicrobials”.
[1] Industry guidance for quality, safety, effectiveness, and labelling of food cultures (preparations) are under development by EFFCA members.
[2] EFFCA, Definition of Food Cultures (updated January 2023)
[3] For bacterial strains belonging to Enterococcus faecium or Bacillus spp. other tests are required according to EFSA 2018 Guidance.
[4] Term officially used by EFSA since 2012 – please see further references in the EFSA 2018 guidance referred to in the document.
[5] Disclaimer: each company remains responsible to determine the safety of a strain and its products.


